Synopsis of project aims
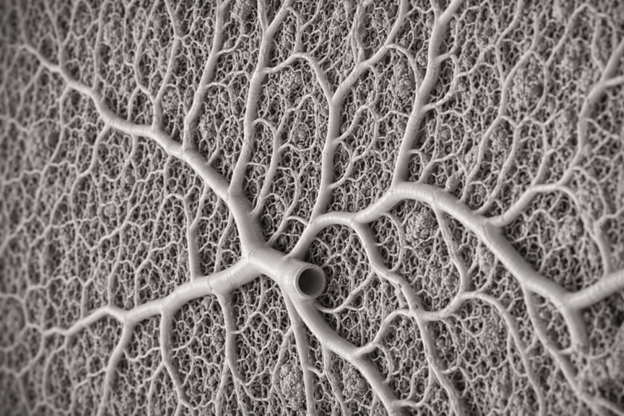
This project aims to develop a scalable biofabrication platform for large-scale, perfusable SLA-printed constructs, addressing one of the central bottlenecks in tissue engineering: maintaining structural fidelity, perfusion, and mass transport as construct size increases. By combining AI-driven STL generation, high-resolution SLA bioprinting, and optical coherence tomography (OCT)-based perfusion imaging, the platform is designed to support the fabrication and validation of complex, vascularized architectures at scales not accessible with conventional design and evaluation workflows.
A key innovation of this effort is the use of gigabyte-scale STL files generated through an agentic AI design pipeline. These files encode dense, hierarchical channel networks and multi-scale geometries required for perfusable constructs, but which exceed the practical limits of traditional CAD-based design approaches. Our workflow is designed to enable the generation, handling, and iterative refinement of these large STL files for bioprinting, linking design complexity directly to functional readouts.
To validate perfusability and structural integrity, we are building an integrated OCT-based imaging and analysis platform that allows real-time visualization of flow through printed constructs. By correlating OCT-derived perfusion maps with AI-generated design parameters, the system is designed to create a closed-loop pipeline in which imaging feedback informs subsequent STL generation and refinement.
Why This Matters for Scale
As bioprinted constructs increase in size and complexity, failures in perfusion, channel collapse, or unintended occlusions become increasingly difficult to detect with endpoint assays alone. This platform is designed to address these challenges by enabling non-destructive, volumetric assessment of perfusion and by tightly coupling design, fabrication, and validation. The result is a scalable framework for producing and evaluating perfusable constructs suitable foradvanced in-vitro models and future tissue-scale applications.
Project Team
- Gilad Gome
- Liam Aranda-Michel
- Ginger Schmidt
- Roy Siegelmann
- Erick Gross
- Brett Bouma
- Ron Weiss
- Tolga Durak